ISKO
Why ISKO?
Because it allows BES studies to be carried out without paperwork. What’s more – ISKO is the backbone of our CT-related product family and using ISKO gives you additional possibilities through the integration of other systems such as remote monitoring etc.
Ask for a demo:
This is only a brief description of a complex system. Please contact us if you like more information.
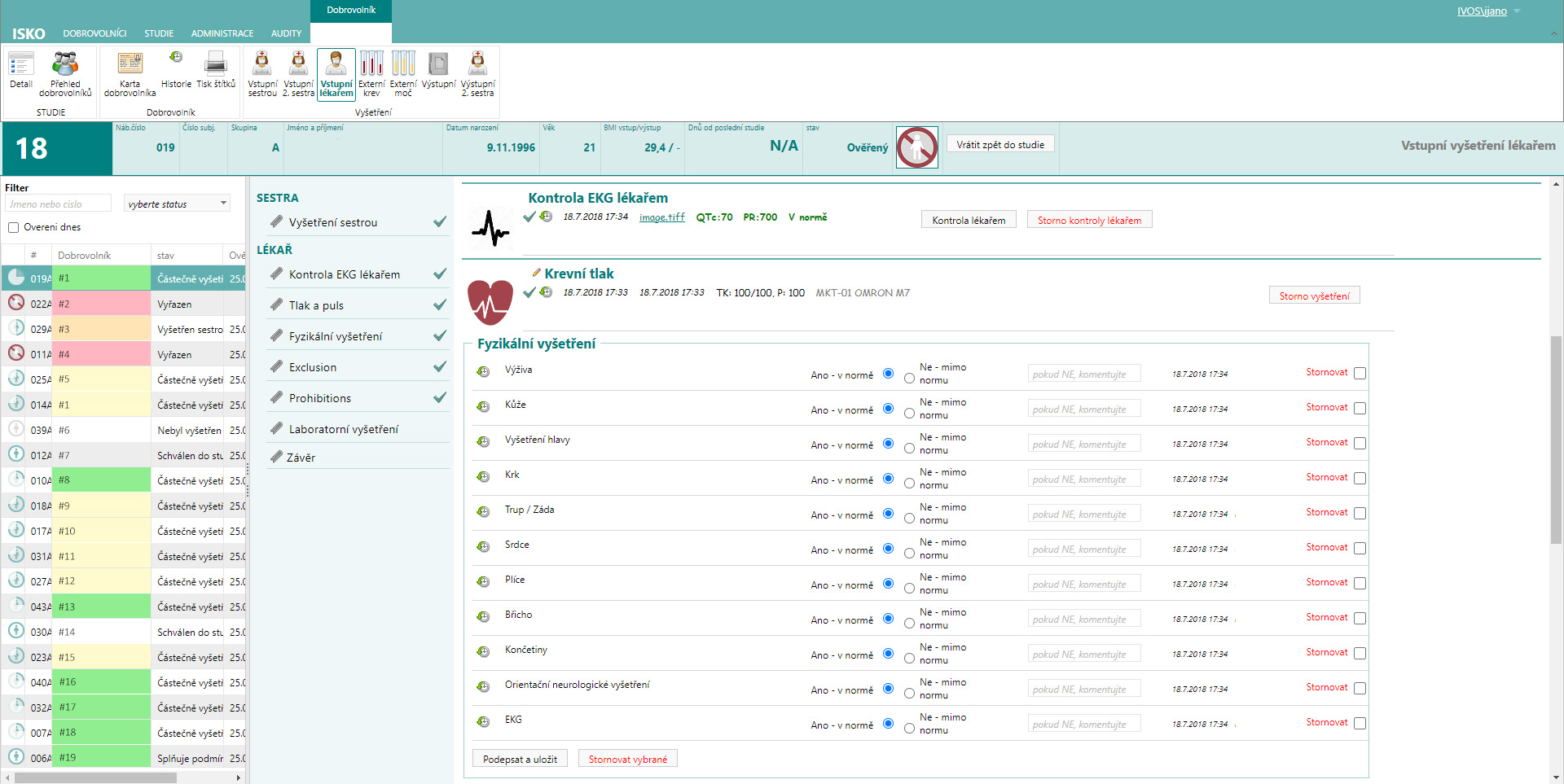
What is ISKO
ISKO is a system for electronic collection of study data without the need to keep paper data. The system contains 3 basic parts:
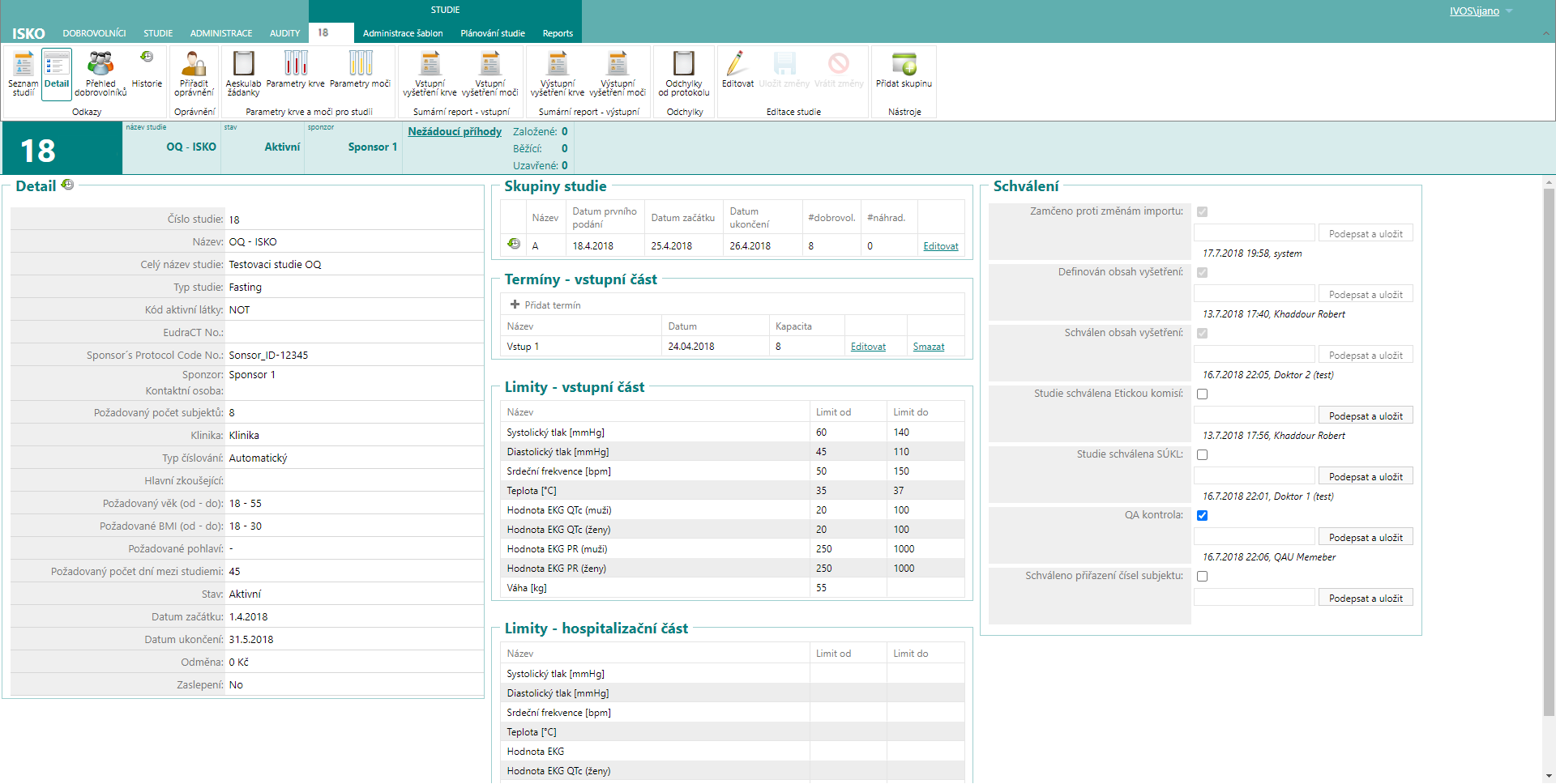
Study definition
In this part, the exact flow of the study can be defined – groups, phases, volunteers, entry and exit examinations, inclusion and exclusion criteria, study entry parameters.
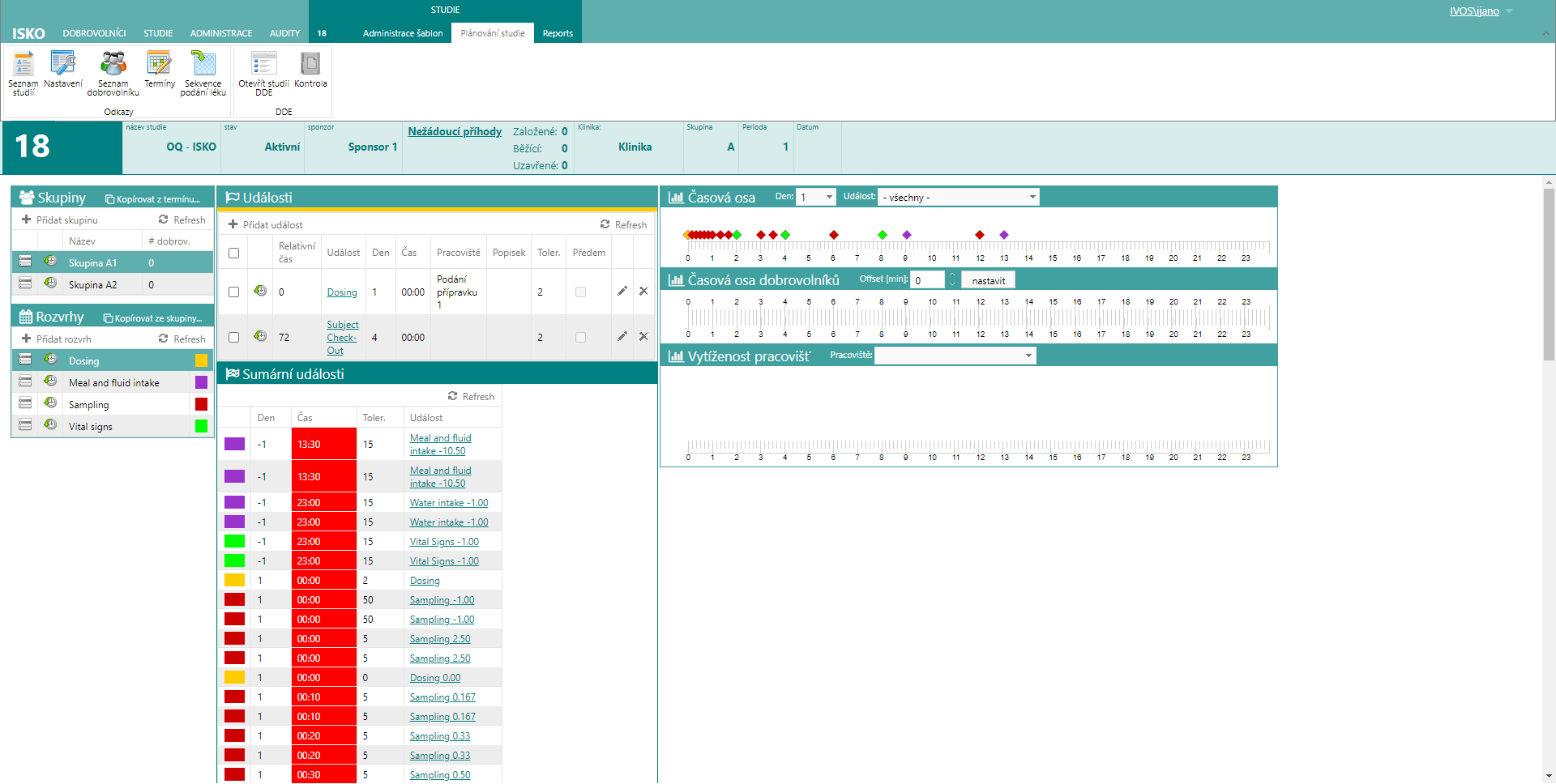
And then the study flow itself, where the time sequence of all events for a sample volunteer is defined. For example, dosing, meals, fluid intake, sampling, testing, and so on.
The system then generates the exact schedule for each volunteer and the capacity requirements for each site.
Another key component of the system is the actual execution of the study.
The system organizes volunteers according to the given schedules so that they are always on time at the right workplace – make use of large screens in common areas or voice commands, for example.
At each workplace there is a device to record the activity and its outcome and comment on any anomalies. These devices are different for different workplaces to make the system as efficient as possible. In some places it is a laptop or an iPad – for example for dosing, where two people are usually needed to check independently. Elsewhere it’s just an RFID reader – for example, for collection, where the record is made automatically by scanning the volunteer’s RFID chip.
The data is thus recorded directly during the study.
Reporting
The last important part of the system is reporting. This allows the generation of all important study reports in a predefined form. Reporting the study, which is usually a challenging activity, can thus be done practically in a few clicks.
In addition to the traditional reports, the system allows reporting data directly in cDISC format and sending them directly to the appropriate authority (typically the FDA).
But there is more …
Another important function of the system is the continuous sending of data to an external server. This ensures that the data has not been tampered with in any way, even at system or database level.
The exported data can also be used for online remote monitoring of the study by the sponsor and thus significantly improve the efficiency of on-site monitoring.
ISKO – basic study planning
ISKO – time schedule study planning